Ebb Medical in Sweden
Ebb Medical is a Swedish pharmaceutical company working with parallel import of original and generic products to the Swedish market.
About us
Ebb Medical is a Swedish pharmaceutical company established 2011 in Karlskrona (southern Sweden). Ebb Medical has since 2011 a wholesale distribution license issued by the Swedish national authority – The Medical Products Agency.
We provide the Swedish pharmacies with parallel imported/distributed prescription drugs to a lower price than the corresponding approved product, the so called direct imported product. We also participate in public tenders for pharmaceuticals used in hospitals and primary care centers. Our products are distributed by Tamro.

Ebb Medical is a shareholder of the Swedish Pharmaceutical Insurance (Läkemedelsförsäkringen). This ensures that patients using our pharmaceutical product and suffers a medication-related illness or injury have the right to have his/her injury investigated by the Swedish Pharmaceutical Insurance Service and if relevant, receive compensation from the insurance.
Ebb Medical is a member of the Swedish Association of Pharma Traders (Läkemedelshandlarna). Many of the issues are decided at European level. Therefore, the Swedish Association of Pharma Traders are members of Affordable Medicines Europe, who represents Europe’s licensed parallel distribution industry.
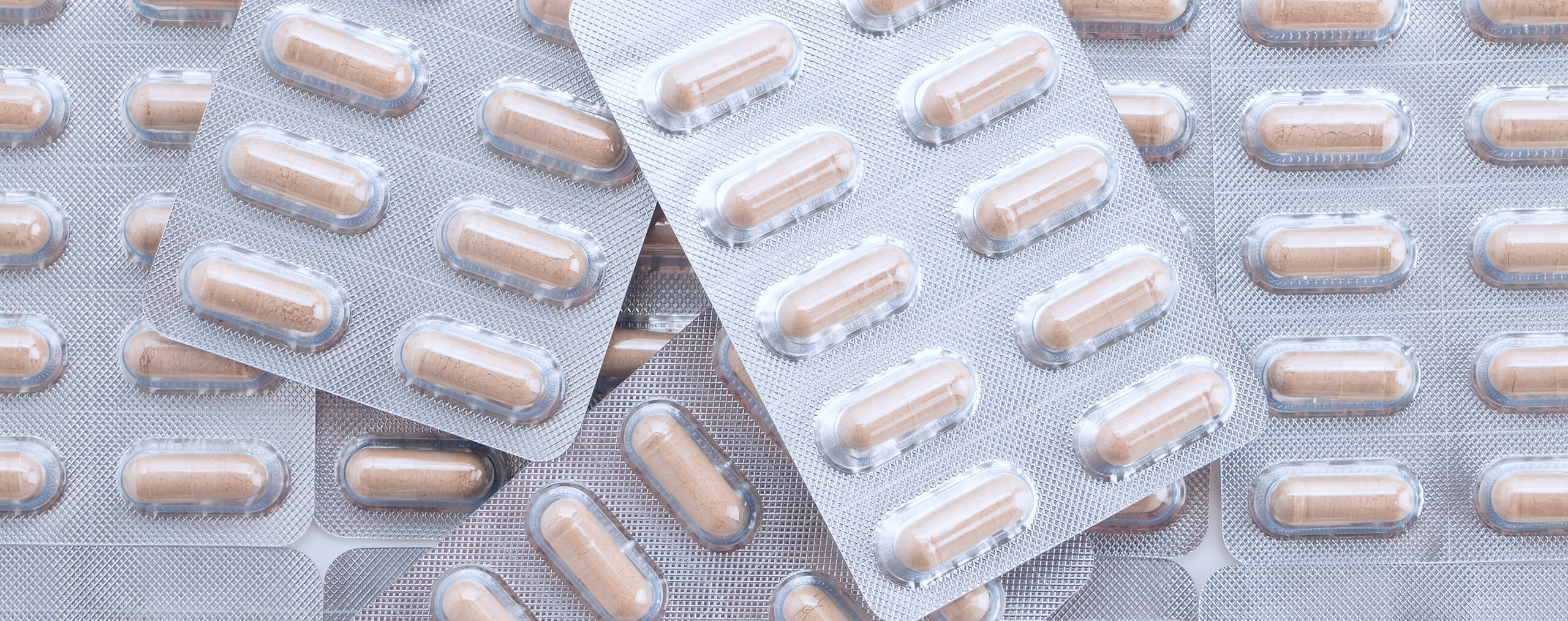
Parallel imports

Parallel import is based on the free movement of medicines within EU/EEA. The prices of medicinal products differ between the countries within EU/EEA due to the individual market conditions in each country. Parallel import is the process of sourcing original medicinal products from a country where the price is lower and then selling them in a different country where the price is higher.
Ebb Medical purchase original products or generic products from authorised wholesalers. The products are repacked into Swedish labelled packages together with a Swedish patient information leaflet before they are sold on the Swedish market. The repackaging process is performed by an authorised manufacturer with a valid manufacturing license (GMP license) issued by the national authority.
All medicinal products marketed in Sweden by Ebb Medical must be approved by the Medical Product Agency or the corresponding European Medicines Agency.
More information
Swedish Pharmaceutical Insurance
The Swedish Pharmaceutical Insurance covers anyone who is treated with prescribed medication or medication purchased from a legitimate dealer in Sweden. It also includes patients who receive medication at a hospital, or who suffer adverse reactions or side effects due to participation in clinical trials that are covered by the insurance.
TLV
The Dental and Pharmaceutical Benefits Agency, TLV, is a central government agency whose remit is to determine whether a pharmaceutical product, medical device or dental care procedure shall be subsidized by the state. They also determine retail margins for all pharmacies in Sweden, regulate the substitution of medicines at the pharmacies and supervise certain areas of the pharmaceutical market.
European Medicines Agency
The European Medicines Agency (EMA) is a decentralised agency of the European Union (EU), located in London. It began operating in 1995. The Agency is responsible for the scientific evaluation, supervision and safety monitoring of medicines in the EU.
Läkemedelshandlarna
Läkemedelshandlarna (the Swedish Association of Pharma Traders) is the organisation for parallel import in Sweden. Ebb Medical is one of their members.
Affordable Medicines Europe
Affordable Medicines Europe represents Europe’s licensed parallel distribution industry.
Contact information
Head office
Postal address: Box 114, 371 22
Karlskrona, Sweden
Visiting address: Östra
Köpmansgatan 36, 371 32 Karlskrona, Sweden
Urgent medical matters from healthcare outside working hours are referred to the on-call telephone at Falck Ambulans, telephone number:
In case of medical questions, we refer to:
Läkemedelsupplysningen, phone number:
Sjukvårdsrådgivningen, phone number:
Suspected adverse reactions, events and incidents should be reported to the Swedish Medical Products Agency:
Our products are insured by Swedish Medicine Insurance (Svenska Läkemedelsförsäkringen), telephone number:
For other questions, please contact us by filling in the contact form below or send an e-mail to:
Please note:
As a pharmaceutical company, we are not allowed to give individual advice on medical treatment. These questions must be answered by a doctor/healthcare professional.
We do not have any information about stock levels at the pharmacies. For this, we refer directly to the respective pharmacy chain.
Contact form
Please fill in the form below and we will get back to you as soon as possible.
